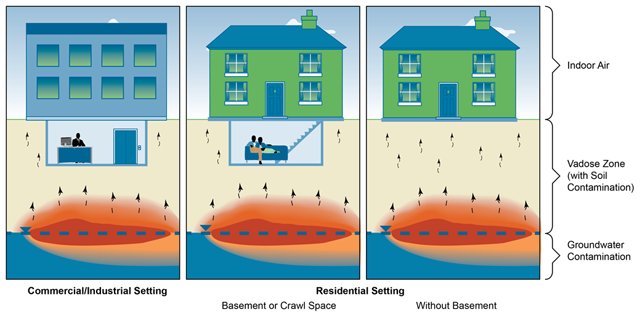
VI occurs when vapors from contaminated groundwater or other subsurface sources migrate upward through vadose zone soils and into overlying buildings. Figure 2-1 depicts a general CSM for the VI process. A CSM is a summary of site-specific conditions and describes the relationship between contaminant sources, contaminated media, migration pathways, and potential receptors.

General conceptual site model for the VI pathway.
Some vapor effects in indoor air are not related to the VI pathway. Examples of these effects include:
Most of the available guidance on VI has focused on contamination from CVOCs, such as tetrachloroethene (dry cleaning fluid). PHCs have more recently been a topic of interest because of recent advances in the science. While PVI has similarities to chlorinated vapor intrusion (CVI), recent research and analysis has increased the understanding of the significant differences between PVI and CVI.
The defining feature of PVI that distinguishes it from VI of other volatile chemicals, most notably CVOCs, is the relatively rapid rate of attenuation of petroleum hydrocarbons (PHCs) because of aerobic biodegradation in vadose zone soils.
Many studies have documented the subsurface biodegradation of PHC vapors (McAlary et al. 2007; Ririe, Sweeney, and Daugherty 2002; Hers et al. 2000b; Ostendorf et al. 2000). Recent evaluations (USEPA 2013i; Lahvis et al. 2013a; Davis 2009) of empirical soil gas data have demonstrated that biodegradation can limit the migration of PHC vapors from a subsurface source. These studies indicate that the potential for PVI is reduced because biodegradation minimizes the flux of PHC vapors in soil gas from a source to overlying buildings. Although PVI may be possible under certain environmental conditions, McHugh et al. (2010) note that “the most common cause of petroleum vapor intrusion is dissolved PHCs or LNAPL in direct contact with building structures such as sumps, basements, or elevator pits.”
Table 2-1 summarizes key differences between PVI and CVI. Much of the information is presented in the document Petroleum Hydrocarbons and Chlorinated Hydrocarbons Differ in Their Potential for Vapor Intrusion (USEPA 2012g). These differences form the basis for the PVI-specific site screening approach, as introduced in
|
Property |
PHCs |
CVOCs |
PVI-related details |
|---|---|---|---|
|
Distribution in groundwater |
A significant portion of the source mass can reside above the water table as LNAPL. |
The majority of free- phase product (DNAPL) migrates below the water table to a less penetrable layer. |
|
|
Biodegradation |
Primarily aerobic; relatively rapid; biodegradation interface is small (from a few inches up to 5 or 6 feet) – see Figure 3-1
|
Primarily anaerobic (except vinyl chloride); relatively slow; generally limited to anoxic zones |
|
|
Biodegradation products |
Aerobic conditions: carbon dioxide (CO2) and water. Anaerobic conditions: methane and carbon monoxide (CO). |
Degradation chemicals generally are toxic. |
|
The following fate and transport mechanisms explain the behavior of PHC vapors and describe how this behavior affects the PVI pathway:
Biodegradation, discussed below, is of critical importance for understanding PVI and is the basis for the site screening strategy presented in Chapter 3. The processes of partitioning, diffusion, advection, and mixing are the same for PHCs and other compounds, including CVOCs. Further details on these processes and the biogeochemical behavior of PHCs are discussed in Appendix M, Fate and Transport of Petroleum Vapors, and Appendix C, Chemistry of Petroleum.
Biodegradation
PHC-degrading bacteria are found in all environments and can consume hydrocarbons rapidly in the presence of O2. This activity can limit transport and VI effects of PHC vapors.
Biodegradation is the breakdown of organic chemicals, including PHCs, by microorganisms. Microorganisms that biodegrade PHCs are ubiquitous in most subsurface soils. Although PHCs can be biodegraded in the absence of O2, the most rapid rates of biodegradation typically occur under aerobic conditions. The vadose zone above an area contaminated by a petroleum release is normally an aerobic environment in which O2 can be readily replenished from the atmosphere. Because rates of petroleum vapor biodegradation usually exceed the rates of petroleum transport via diffusion, petroleum vapors are typically, but not always, fully attenuated by aerobic biodegradation processes in the vadose zone.
A notable feature of aerobic biodegradation of PHCs in soils is the short acclimation time for this process, which can be measured in hours and days (Turner et al. 2014). The acclimation time is the time required for the microbial community to start consuming PHCs after the initial introduction of these chemicals. This short acclimation time indicates that PHC biodegradation is a common physiological trait of soil microorganisms.
Despite the general reliability of aerobic biodegradation in reducing PVI, several environmental factors can hinder this process. The most significant factor is the availability of O2, which is a necessary electron acceptor and enzyme reactant in the aerobic biodegradation of PHCs. Some state regulations/guidance documents indicate that O2 levels only need to be greater than 2–4% by volume to support aerobic biodegradation. In addition, Roggemans, Bruce, and Roggemans (2001) showed oxygen concentrations of 2% by volume to be supportive of aerobic biodegradation.
Acclimation of Microorganisms
Microbial communities can start consuming PHCs within hours or days of the introduction of PHCs into the subsurface.
Some factors that may hinder the recharge of O2 in the vadose zone are soils with high moisture content; soils with high organic content; soils with low permeability; large building foundations; and the presence of high PHC concentrations, such as near LNAPL sources, that consume O2 from biodegradation itself. In the absence of O2, anaerobic microorganisms can use other electron acceptors to support PHC biodegradation. Anaerobic biodegradation of PHCs is typically slower than aerobic biodegradation, and the rates of biodegradation in the presence and absence of O2 can differ substantially.
Other factors that can potentially limit the biodegradation of PHCs include low moisture content, nutrient availability, temperature, and heavy metals. In the vadose zone, sufficient moisture and nutrients usually are present to support microbial PHC biodegradation. However, in more extreme circumstances (such as arid environments), insufficient moisture can potentially limit PHC biodegradation even when sufficient O2 is available. In general, the rates of biological processes decrease with decreasing temperature. Cold climates, however, do not preclude the potential for biodegradation because some specific PHC-consuming microorganisms thrive in temperatures ranging from 20ºC to 0ºC (Margesin and Schinner 2001). Similarly, heavy metal contaminants can be toxic to PHC-degrading bacteria and can decrease PHC degradation rates (Babich and Stotzky 1985). Many microorganisms, however, have the capability to resist the toxic effects of heavy metals and have high activity in environments affected by these common inorganic contaminants.
Methane Production
Methane may be produced when O2 is depleted in the presence of high PHC concentrations or large source areas, or by the breakdown of petroleum products containing ethanol.
When PHCs are present at sufficiently high concentrations or in large source areas, O2 and other electron acceptors may become depleted. PHCs may then be biodegraded through the activities of methanogenic microbial communities. Because methane is not a component of gasoline or other liquid hydrocarbon products, the presence of methane indicates that insufficient O2 is available for aerobic PHC biodegradation.
The presence of methane can also further affect PHC biodegradation, because methane itself can be readily biodegraded under aerobic conditions. The consumption of O2 for methane biodegradation can limit the amount of O2 available for biodegradation of other PHCs. O2 levels can also be reduced in the presence of ethanol by a similar mechanism, resulting in further methane production. However, the effect of methanogenic degradation of ethanol in this context is limited in fuels containing 10% or less ethanol by volume (Wilson et al. 2012; Wilson et al. 2013). The vertical screening distances discussed in Chapter 3 were developed in part from empirical site data for fuel releases that included 10% ethanol.
The chemical structures of PHCs and their physicochemical properties can also influence their biodegradation. Generally, for PHCs dissolved in the water phase, microorganisms biodegrade n-alkanes (straight-chain alkanes) more rapidly than cyclic and aromatic compounds, and biodegrade shorter chain n-alkanes more slowly than longer chain n-alkanes (Alexander 1977). The structure of the chemical (for example, more branching) and the presence of specific substituents or functional groups (for example, the ether group on methyl tert-butyl ether, or MTBE) can also strongly affect biodegradability.
Another important factor that affects the biodegradability of a chemical based on its structure is the air-to-water partitioning coefficient. For petroleum chemicals, because the air-to-water partitioning of aromatic compounds is less than n-alkanes, a greater fraction of the aromatic compounds are partitioned into water, are more readily available to be biodegraded, and therefore may be more significantly attenuated by microbial biodegradation than n-alkanes (DeVaull 2007).
A summary of the effects of chemical structure on aerobic hydrocarbon biodegradation rates is presented in
A CSM is a visualization of site conditions that allows for evaluation of contaminant sources and affected media, migration pathways, and potential receptors. This tool provides an iterative representation of the site and guides decision making while assessing the PVI pathway.
A CSM for PVI incorporates biodegradation and is used to determine whether a complete PVI pathway exists or, if needed, the information required to make this determination. Information to construct the CSM is acquired from historical research, site characterization, and an understanding of contaminant behavior, among other sources. The CSM is a dynamic tool that should be refined throughout the life of the project as new information is acquired.
An example of a CSM for the PVI pathway is shown in Figure 2-2. The CSM for a site with a potential for PVI will evolve as the assessment progresses through the assessment strategy summarized in Figure 1-2. However, in order to use the screening method described in Chapter 3, only a limited set of key information and data (what is termed a "preliminary CSM"), must be compiled.
Appendix D provides a checklist to assist in the development of a CSM for the PVI pathway.
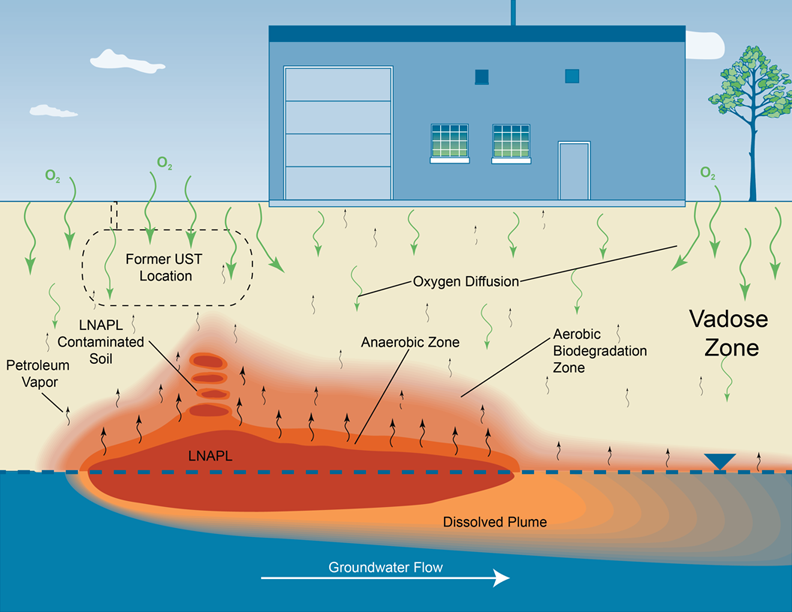
General conceptual site model for the PVI pathway.
Understanding and properly characterizing the petroleum source is an important part of the CSM for PVI. Petroleum fuels can be broadly categorized as “gasolines,” “middle distillates,” and “residual fuels,” with the middle category including diesel, kerosene, Stoddard solvent and some types of jet fuels (API 1994). The general makeup of common petroleum fuels in terms of the number of carbon molecules per compound is depicted in Figure 2-3. The detailed chemistry of petroleum fuels has been extensively studied (Potter and Simmons 1998, USEPA 2009f). These fuels are primarily composed of hundreds of nonspecific, aliphatic hydrocarbon compounds with a small, variable amount of aromatic compounds, including BTEX and naphthalene. Collectively, all of these hydrocarbons are referred to as “Total Petroleum Hydrocarbons” (TPH). The vapors associated with petroleum fuels are similarly dominated by aliphatic compounds with smaller amounts of aromatic compounds (USEPA 2013a, Brewer et al. 2013). The actual vapors above the petroleum source will depend on the constituent composition of the vapor source, vapor pressures of the constituents, and temperature. A more detailed overview of the chemistry of petroleum and its vapors is provided in Appendix C.
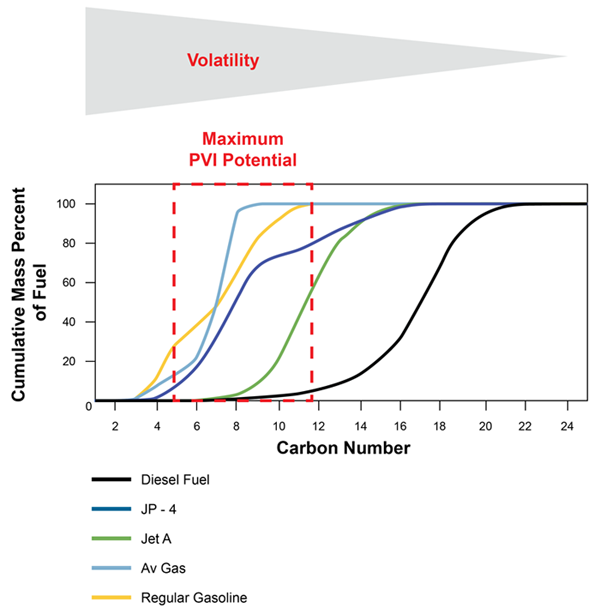
Composition of typical petroleum fuels with respect to the number of carbon molecules in individual compounds.
Petroleum products may be released to the environment at industrial, commercial, and residential properties. The type of site and PHC, as well as the nature of the release and subsurface lithology, influences the distribution of PHC in the subsurface.
The ITRC PVI survey of 49 states and the District of Columbia identified nine common types of petroleum release sites. Table 2-2 summarizes common types of petroleum sites and general features of each that may be related to the potential for PVI. The site type examples detailed in Table 2-2 are examples of common petroleum site types and may not cover all site type possibilities or all site-specific scenarios. Components of the sites listed may not be applicable to other types of petroleum sites. The characteristics summarized in Table 2-2 are characteristics as they relate to the potential for PVI. For example, the indicator compounds listed in the Table are compounds that may be important in evaluating the potential for PVI. The indicator compounds summarized are not an exhaustive list of possible compounds that may be detected on the particular petroleum site type.
The discussion of indicator compounds for PVI generally focuses on a small number of individual, well-studied, aromatic compounds such as benzene, toluene, ethylbenzene, xylenes, and naphthalene that are traditionally considered in human health risk assessments. The role of nonspecific, petroleum-related aliphatic and aromatic compounds in PVI has only recently come under more scrutiny (for example, Brewer et al. 2013). These compounds are collectively measured and assessed in terms of TPH or in more detail as specific groups of aliphatic and aromatic carbon ranges (such as C5–C8 aliphatics, C9–C12 aliphatics, or C9–C18 aromatics). Some regulatory agencies (for example, Hawaii Department of Health) may require a more detailed evaluation of the role of TPH in PVI. Approaches for providing these detailed evaluations, however, are beyond the scope of this document. Refer to the local regulatory agency for further guidance.
|
Petroleum site type (link to Appendix E) |
Common indicator compounds |
Potential release sources |
|---|---|---|
|
Gasoline: BTEX, trimethylbenzenes, naphthalene, methane Diesel: naphthalene, methane |
USTs, product lines, dispensers, service bays |
|
|
Naphthalene, benzene |
USTs, ASTs, product lines |
|
|
BTEX, naphthalene, methane |
Underground or aboveground piping, USTs (former and current), ASTs, loading areas, tank pits (current and former), processing units, historical disposal sites |
|
|
For oil/petroleum/gasoline: BTEX, naphthalene, methane |
Underground or aboveground piping, ASTs, oil/water separators, loading areas |
|
|
For oil/petroleum: BTEX, naphthalene, methane For natural gas: methane, butane, propane, benzene |
Pipeline, pipe joints, valves, flanges, weld points
|
|
|
BTEX, methane |
Wells and well area, pipelines, gathering lines, mud pits, USTs and associated piping, ASTs and associated piping, maintenance facilities, oil/water separators |
|
|
BTEX, indane, indene, naphthalene, trimethylbenzenes |
Tar holders, oil/water separators, gas holder foundations, purifying boxes, tar wells |
|
|
Naphthalene, alkyl-naphthalene derivatives, benzene |
Drip pads, product storage areas, unlined pits, lagoons |
|
|
BTEX |
Outside building (especially windows and doors), storage areas, dry wells, drains |
Additional detailed information about common types of petroleum sites is contained in Appendix E, Common Types of Petroleum Sites. Site type information includes: